

A Prediction Model of Evaporation Behavior of Nickel-Based Superalloy in Electron Beam Melting
(School of Materials Science and Engineering, Dalian University of Technology, Dalian 116024, China)
摘要: The electron beam melting technique has emerged as a novel refining technology extensively employed for alloy purification due to its advantages of effectively eliminating volatile impurities and non-metallic inclusions. However, a drawback associated with the melting process is the evaporation of alloy elements, which results in an uncontrollable alloy composition. In this paper, Wagner model is used to calculate and study the evaporation behavior. of nickel-based superalloy, and the database of infinite dilution activity coefficient and interaction coefficient of nickelbased superalloy has been established. The results show that the model can well predict the composition of electron beam melting superalloy, establish the relationship between electron beam melting power and temperature, and predict the composition of several groups of DZ125 alloys after melting with different power, which is in consistent with the experimental results. This model is of guiding significance for electron beam melting of nickel-based superalloy.
DOI: 10.48014/pcms.20231010001
引用格式: DONG Gengyi, YIJIALA Yiliti, HAN Wenjun, et al. A prediction model of evaporation behavior of nickel-based superalloy in electron beam melting[J]. Progress in Chinese Materials Sciences, 2024, 3(1): 1-11.
文章类型: 研究性论文
收稿日期: 2023-10-10
接收日期: 2023-11-27
出版日期: 2024-03-28
1 Introduction
The utilization of nickel-based superalloys has been prevalent in contemporary aero-engines,establishing them as the primary material choice[1,2].These alloys are also widely employed as structural components in advanced aero-engines,constituting approximately 40% of the total weight.This preference is primarily attributed to their remarkable mechanical properties and resistance to corrosion when exposed to elevated temperatures[3,4].There is an increasing requirement for strict material characteristics in the development of aero-engines with high thrust-to-weight ratios.Prior studies have shown that the presence of inclusions and impurity elements exerts a substantial detrimental effect on the properties and attributes of nickel-based superalloys.The regulation of the composition of inclusions and impurity elements is of utmost importance in determining the properties and reliability of superalloys[5,6].
Currently,the predominant techniques employed for the fabrication of superalloys includeelectroslag remelting,vacuum induction melting,and vacuum arc melting.The integration of these technologies is extensively employed in the fabrication of superalloys[7].The aforementioned techniques have the capability to eliminate a majority of inclusions;nonetheless,a minority of inclusions can still have an impact on the characteristics of alloys.Consequently,an increasing number of researchers are endeavoring to manipulate the composition of inclusions within the alloy through modifications to the melting process and melting mode.However,it remains a challenge to eliminate small inclusions measuring less than 10μm[8-10].Electron beam melting(EBM)is a very efficient technique utilized for the process of melting alloys.However,this purification process also results in varying degrees of alloy element loss.The volatilization of alloying elements has an impact on whether the alloy composition falls within the desired range,and precise regulation of the chemical composition of the alloy is also a significant factor influencing the operational effectiveness of the alloy.Our research group has previously developed several models for estimating the evaporation of alloy components.The Miedema model to assess the evaporation behaviors of Inconel 718,GH4068,and FGH4096 alloys have been used in our study.However,it is worth noting that only the primary elements,which constitute the majority of the alloys,were considered in the calculations[11-14].Consequently,the Wanger model was employed in the previous study to determine the melting loss of the DD98M alloy.The obtained results exhibited a high level of concurrence with the experimental findings[15].Nevertheless,this particular model is not limited to the evaluation of the evaporation behavior just in DD98M alloy.It may also be employed to analyze other alloys and effectively regulate their composition.
In this paper,a comprehensive database has been developed to document the interaction coefficient and infinite dilution activity coefficient of elements in nickel-based superalloys.Thus,the loss behavior of various superalloys has been computed.The validity of the model is confirmed through experimental verification,demonstrating its applicability to the evaporation process in electron beam melting.Additionally,the model establishes a correlation between power and temperature in electron beam melting,offering theoretical insights for the fabrication of nickel-based superalloys using this technique.
2 Alloy evaporation model
Hertz and Knudsen on the basis of the Langmuir equation,the expression of ideal evaporation rate of i component in vacuum melting process is as follows[16]:
![]() (1)
(1)
![]() (2)
(2)
![]() (3)
(3)
where Pi is the partial vapor pressure of component i in the alloy melt,Mi is the molar mass,R is the gas constant,which is taken 8.31J·mol-1·K-1,T is the alloy melt temperature,ai and ![]() are the activity and the activity coefficient of component i,
are the activity and the activity coefficient of component i,![]() is the saturation vapor pressure of the pure component i,and
is the saturation vapor pressure of the pure component i,and ![]() is the molar fraction.
is the molar fraction.
According to Clausius-Clapeyron equation,the saturated vapor pressure of each element of DD98M alloy is calculated as follows:
![]() (4)
(4)
where the correlation coefficients are shown in Table 1.The calculated relationships between the saturated vapor pressure of each element in superalloy and the melt temperature are shown in Fig.1(a).The Fig.1 illustrates that the evaporation rates of Ni,Cr,Al,and Co elements in superalloy are significantly larger compared to other elements within the temperature range of 1500 K to 2500 K,which serves as the primary cause of mass loss during the EBS.
Table 1 Parameters of saturated vapor pressure for elements[17]
|
Element |
A |
B |
C×1000 |
D |
Range/K |
|
Al |
-16380 |
-1.00 |
— |
12.32 |
933-2600 |
|
Cr |
-20680 |
-1.31 |
— |
14.56 |
298-2180 |
|
Co |
-22209 |
— |
-0.223 |
10.82 |
298-1768 |
|
Fe |
-21080 |
-2.14 |
— |
16.89 |
298-1265 |
|
Fe |
-19710 |
-1.27 |
— |
13.27 |
1265-2588 |
|
Hf |
-29830 |
— |
— |
11.32 |
2503-4723 |
|
Mo |
-34700 |
-0.24 |
-0.15 |
11.66 |
298-2893 |
|
Nb |
-37650 |
+0.715 |
-0.166 |
8.94 |
298-2195 |
|
Ni |
-22500 |
-0.96 |
— |
13.60 |
298-1728 |
|
Ni |
-22400 |
-2.01 |
— |
16.95 |
1728-3187 |
|
Re |
-40800 |
-1.16 |
— |
14.2 |
298-3000 |
|
Ta |
-40800 |
— |
— |
10.29 |
298-3269 |
|
Ti |
-24400 |
-0.91 |
— |
13.18 |
1155-1933 |
|
Ti |
-23200 |
-0.66 |
— |
11.74 |
1933-3560 |
|
W |
-44000 |
+0.50 |
— |
8.76 |
298-3683 |
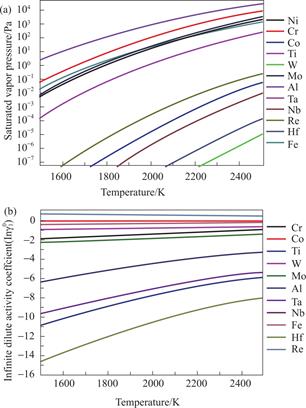
Fig.1 The variation of (a) the saturated vapor pressure, (b)the activity coefficient of a solute in infinite dilute solution of elements in superalloy with melt temperature
Since the Wagners theory is only effective in the strict sense of dilute solution,Pelton and Bale are modified on the basis of it,and the activity coefficient in solution can be calculated,as follows[18]:
![]()
![]() (5)
(5)
where ![]() is the activity coefficient of a solute in infinite dilute solution of component i,and
is the activity coefficient of a solute in infinite dilute solution of component i,and ![]() is the activity interaction parameters of component j on component i.
is the activity interaction parameters of component j on component i.
The activity coefficient of a solute in infinite dilute solution and the activity interaction parameters were established by previous research[15],and the specific calculation equation is as follows:
![]() (6)
(6)
where:
![]()
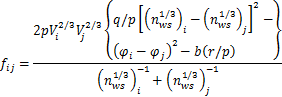
![]() (7)
(7)
where:
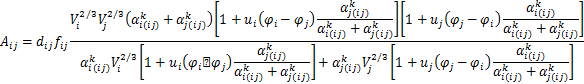
![]()
![]()
![]()
![]()
where ![]() 、Tmi、(
、Tmi、(![]() )i and φi are the molar volumes、the melting points、the electron densities and the electroneg ativities of component i,respectively,and p,q,u,b and r/p are the correlation constants(q/p = 9.4;b = 0.73).The values of other parameters can be found in Table 2.
)i and φi are the molar volumes、the melting points、the electron densities and the electroneg ativities of component i,respectively,and p,q,u,b and r/p are the correlation constants(q/p = 9.4;b = 0.73).The values of other parameters can be found in Table 2.
Table 2 The parameters of components in superalloy employed in Miedemas model[19]
|
metal |
Al |
Cr |
Co |
Fe |
Hf |
Mo |
Nb |
Ni |
Re |
Ta |
Ti |
W |
|
|
4.60 |
3.70 |
3.50 |
3.70 |
5.60 |
4.40 |
4.9 |
3.50 |
4.3 |
4.90 |
4.80 |
4.50 |
|
( |
1.39 |
1.73 |
1.75 |
1.77 |
1.43 |
1.77 |
1.62 |
1.75 |
1.86 |
1.63 |
1.47 |
1.81 |
|
φi/V |
4.20 |
4.65 |
5.10 |
4.93 |
3.55 |
4.65 |
4.00 |
5.20 |
5.40 |
4.05 |
3.65 |
4.80 |
|
r/p/V2 |
1.90 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
|
u |
0.07 |
0.07 |
0.07 |
0.07 |
0.04 |
0.04 |
0.04 |
0.04 |
0.04 |
0.04 |
0.04 |
0.04 |
Eleven binary systems including Ni-Al,Ni-Cr,Ni-Co,Ni-Fe,Ni-Hf,Ni-Mo,Ni-Nb,Ni-Re,Ni-Ta,Ni-Ti,and Ni-W are constructed for superalloy.The infinite dilute activity coefficients of each component at different temperatures are shown in Fig.1(b).
For the Ni-based superalloy,121 ternary systems are constructed,as indicated by Ni-X-X(X=Al,Cr,Co,Fe,Hf,Mo,Nb,Re,Ta,Ti,W).The activity interaction coefficients of the elements in superalloys are shown in Fig.2.
The theoretical evaporation rate of alloy elements during electron beam melting is given by Eq.(8).The theoretical mass fraction of the alloy elements after electron beam melting can be derived by Eq.(9):
![]() (8)
(8)
![]() (9)
(9)
where Δcmi is the theoretical mass loss of element i, S is the surface area of the melt,t is the evaporation time,Vi is the theoretical evaporation rate,[wt.%i] is the mass fraction,mi is the mass of element i before melting,malloy is the mass of alloy before melting,![]() is the mass loss of the alloy.
is the mass loss of the alloy.
3 Electron beam melting experiment
Different Ni-based superalloys were melted by SEM60A equipment.During the experiment,the vacuum in the melting chamber and the electron gun chamber was lower than 8×10-3 Pa,and the alloys of DD98M,GH4068 and FGH4096 were melted by a 76 mm hemispherical crucible(melting mass was 500 g).The melting power is 8-12 kW,and the melting time is 10 min.The specific melting process is shown in the Fig.3(a).The alloy ingot after electron beam melting is shown in the
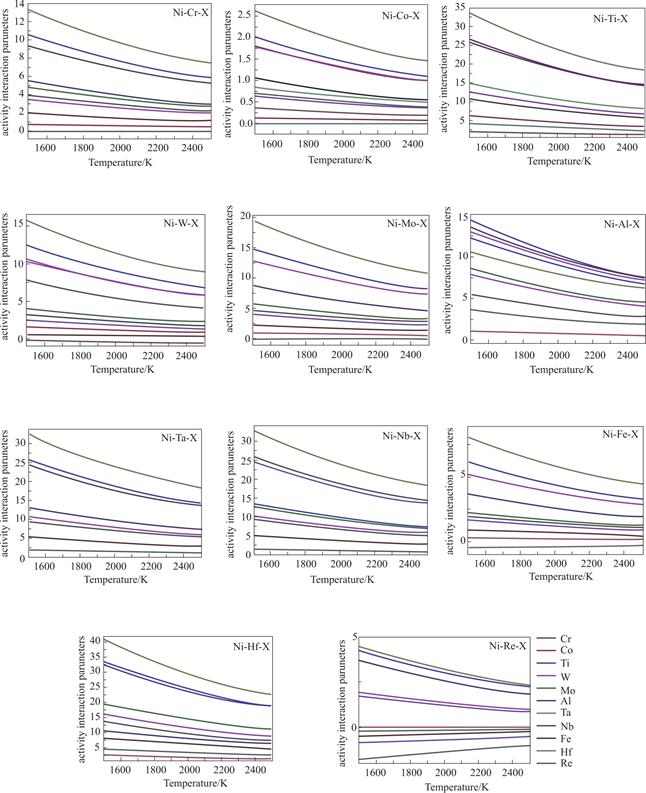
Fig.2 Relationship between activity interaction coefficient of alloy components and temperature
Fig.4(a).Inconel 718,DD5,DD98M and GH4068 alloys were melted in a 120 mm crucible(melting mass was 1500 g).The melting power was between 10 and 16 kW,and the melting time was 10 min.The specific process was the same as before,and EBM ingots were shown in the Fig.4(b).Then,in order to verify the relationship between power and temperature,DZ125 alloy was prepared by different melting parameters.The specific melting process is shown in the Fig.3(b),and the ingot morphology after melting is shown in Fig.4(c).The mass and composition of the alloy before and after melting were measured.
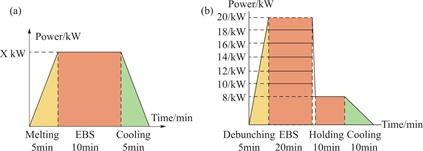
Fig.3 The process flow of electron beam melting

Fig.4 Morphology of ingot after EBS:(a)76 mm crucible;(b)120 mm crucible;(c)DZ125 alloy Discussion
The Fig.5 illustrates both the actual composition and the calculated composition of these alloys.The graphics demonstrate a minimal disparity between the computed composition and the observed composition.During the melting process,the input power to the alloys were 8,10,and 12 kW,which were equivalent to the temperature input to the system of 1855 K,1870 K,and 1912 K,respectively.
The Fig.6 provided displays both the actual composition and the estimated composition of these alloys.The observation from Fig.6 reveals that there is a minimal disparity between the calculated component and the actual component when the mercury level is at 120 mm.The relationships between different alloy melting powers and their corresponding average temperatures are presented in the accompanying table 3.It is evident that the melting power and the average temperature of alloys remain nearly constant when they are melted simultaneously.
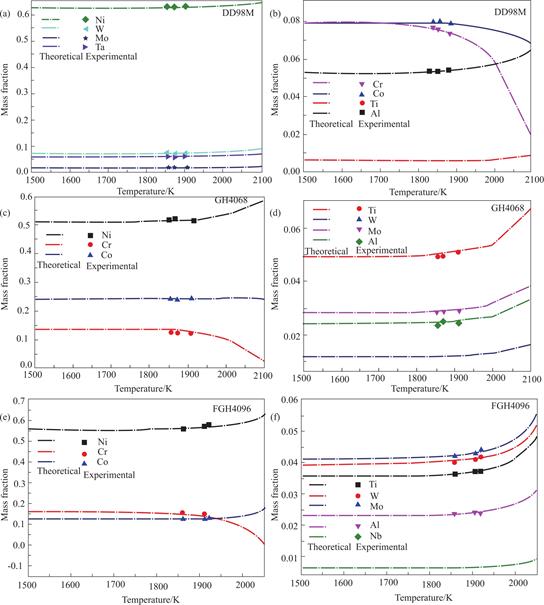
Fig.5 The theoretical and experimental components of different superalloy elements withdifferent temperatures (a)and(b)DD98M;(c)and(d)GH4068;(e)and(f)FGH4096
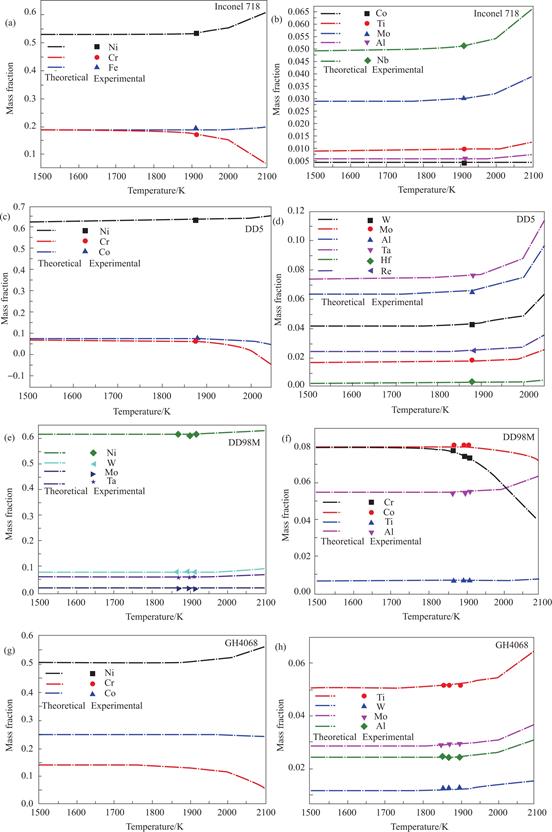
Fig.6 The theoretical and experimental components of different superalloy elements with different temperatures (a)and(b)Inconel 718;(c)and(d)DD5;(e)and(f)DD98M;(g)and(h)GH4068
Table 3 The relationships between different alloy melting powers and their corresponding average temperatures
|
alloy |
P/kW |
T/K |
alloy |
P/kW |
T/K |
|
Inconel 718 |
15 |
1913 |
DD5 |
12 |
1880 |
|
DD98M |
12 |
1870 |
GH4068 |
10 |
1855 |
|
DD98M |
14 |
1901 |
GH4068 |
12 |
1870 |
|
DD98M |
16 |
1911 |
GH4068 |
14 |
1901 |
Based on the derived power values and the mean temperature of the molten surface,it is observed that the crucible dimensions are comparatively smaller and the temperatures are elevated under equivalent power conditions.When the crucible size is determined,there is a positive correlation between the power input and the average surface temperature of the melt.Consequently,a three-dimensional diagram is constructed to visualize the relationship between melting power,the quality of the melting alloy,and the surface temperature of the melt.This diagram is subjected to a fitting process.
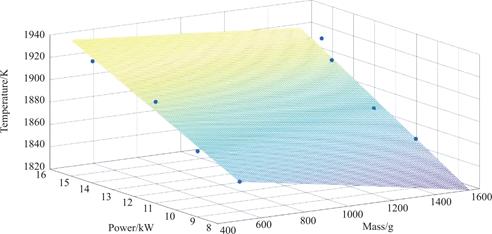
Fig.7 Relationship between different power,alloy quality and average surface temperature of melt during electron beam melting
The relationship between the average surface temperature of the melt and the power is shown in Fig.7,indicating a linear correlation when the melting quality remains constant.To ascertain the correspondence between the power and the surface temperature of the melt,an experiment was conducted using 1500 g of DZ125 alloy.The particular details of the melting process can be observed in the accompanying figure.Currently,it is necessary to compute the mass loss of heat preservation at a power output of 8 kW.These calculations are derived from Eq.9 and Eq.7.
![]() (10)
(10)
where Δcmij is the mass loss during refining,Δc![]() is the mass loss during heat preservation,and the calculation result is as shown in the Fig.7.
is the mass loss during heat preservation,and the calculation result is as shown in the Fig.7.
Based on the observations presented in Fig.8,a clear correspondence can be observed between various melting powers and the anticipated average melt temperature.Furthermore,the alloy composition following the experiment is accurately predicted prior to its execution.These findings offer valuable theoretical insights for effectively regulating the composition of electron beam melting superalloys.
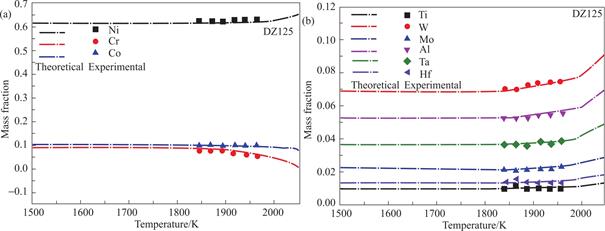
Fig.8 The theoretical and experimental mass fractions of DZ125 superalloy elements in relation with temperature
4 Conclusions
(1)In this experiment,the database of infinite dilution activity coefficient and activity interaction coefficient of each element of nickel-based superalloy was established,and the activity coefficients of different alloys were calculated with the data in the database.
(2)Different kinds of superalloys,including single-crystal superalloys,deformed superalloys and powder superalloys,were melted by electron beam.The melting composition was predicted and was consistent with the experimental results,revealing the evaporation behavior of Ni-based superalloys.
(3)The correlation between the power of electron beam melting and the average surface temperature of the melt has been established,and its validity has been confirmed through experimental analysis,thus demonstrating the credibility of this model.The aforementioned model holds significant implications for the regulation of composition in multi-component superalloys during the process of electron beam melting.
Conflict of interest:The authors declare no conflict of interest.
[①] *通讯作者 Corresponding author:王轶农,wynmm@dlut.edu.cn
收稿日期:2023-10-10; 录用日期:2023-11-27; 发表日期:2024-03-28
基金项目 Funding:This study was supported by the National Key Research and Development Project(Grant No.2019YFA0705300).
参考文献(References)
[1] H. B. Long, S. C. Mao, Y. N. Liu, et al. Microstructural and compositional design of Ni-based single crystalline superalloys― A review[J]. Journal of Alloys and Compounds, 2018, 743: 203-220.
https://doi.org/10.1016/j.jallcom.2018.01.224
[2] J. G. Li, J. X. Sun, J. L. Sun, et al. The precipitation and effect of topologically close-packed phases in Ni-based single crystal superalloys[J]. Journal of Materials Science and Technology, 2024, 173: 149-169.
https://doi.org/10.1016/j.jmst.2023.05.074
[3] Y. Li, Y. Tan, D. G. Wang, et al. Effect of electron beam melt superheating treatment on DZ125 alloy[J]. Journal of Materials Research and Technology, 2020, 24: 6088-6106.
https://doi.org/10.1016/j.jmrt.2023.04.216
[4] Tresa M. Pollock, Sammy Tin, Nickel-Based Superalloys for Advanced Turbine Engines: Chemistry, Microstructure and Properties[J]. Journal of Propulsion and Power, 2006, 22(2): 361-374.
https://doi.org/10.2514/1.18239
[5] L. Zheng, G. Q. Zhang, Michael J. Gorley, et al. Effects of vacuum on gas content, oxide inclusions and mechanical properties of Ni-based superalloy using electron beam button and synchrotron diffraction[J]. Materials & Design, 2021, 207: 109861.
https://doi.org/10.1016/j.matdes.2021.109861
[6] T. T. Zhang, David M. Collins, DUNNE Fionn-P-E, et al. Crystal plasticity and high-resolution electron backscatter diffraction analysis of full-field polycrystal Ni superalloy strains and rotations under thermal loading[J]. Acta Materialia, 2014, 80: 25-38.
https://doi.org/10.1016/j.actamat.2014.07.036
[7] J. Zhang, L. Wang, D. Wang, et al. Recent Progress in Research and Development of Nickel-Based Single Crystal Superalloys[J]. ACTA METALLURGICA SINICA, 2019, 55(9): 1068-1094.(in Chinese)CNKI: SUN: JSXB. 0. 2019-09-003
[8] P. Bai, H. R. Zhang, Y. M. Li, et al. Effect of Y2O3 cruci- 10 中国材料科学进展 Progress in Chinese Materials Sciences ble on purification of Ni3Al-based superalloy scraps[J]. Rare Met. Mater. Eng, 2019, 48(02): 406-410.(in Chinese)
http://en.cnki.com.cn/Article_en/CJFDTotal-COSE201902007.htm
[9] A. J. An, Z. Wang, C. B. Shi, et al. Supergravity-induced separation of oxide and nitride inclusions from Inconel- 718 superalloy melt[J]. ISIJ International, 2019, 60: 206-211.
https://doi.org/10.2355/isijinternational.ISIJINT-2019-321
[10] Mohsen hajipour Manjili, Mohammad Halali. Removal of Non-metallic Inclusions from Nickel Base Superalloys by Electromagnetic Levitation Melting in a Slag[J]. Metallurgical and Materials Transactions B, 2018, 49: 61-68.
https://doi.org/10.1007/s11663-017-1137-z
[11] Q. F. You, S. Shi, X. G. You, et al. Evaporation behavior of Ni, Cr and Fe in Inconel 718 superalloy during electron beam smelting[J]. VACUUM, 2017, 135: 135-141.
https://doi.org/10.1016/j.vacuum.2016.11.012
[12] X. G. You, S. Shi, Y. Tan, et al. The evaporation behavior of alloy elements during electron beam smelting of Inconel 718 alloy[J]. VACUUM, 2019, 169: 108920.
https://doi.org/10.1016/j.vacuum.2019.108920
[13] Q. F. You, Study on preparation of high purity FGH4096 master alloy by electron beam melting and its purification behavior[D]. Dalian: Dalian University of Technology, 2019.
[14] Y. L. Wang, Y. Tan, C. Y. Cui, et al. Evaporation behavior of alloying elements and calculation of molten pool Temperature of electron beam melting of a new Ni-Co based superalloy[J]. Materials Reports, 2023, 37(01): 176-181.(in Chinese)
doi:10.11896/cldb.21080061
[15] G. Y. Dong, X. G. You, Z. H. Xu, et al. A new model for studing the evaporation behavior of alloy elements in DD98M alloy during electron beam smelting[J]. VACUUM, 2022, 195: 110641.
https://doi.org/10.1016/j.vacuum.2021.110641
[16] I. Langmuir. The vapor pressure of metallic tungsten[J]. Phys. Rev, 1913, 2(5): 329-342.
https://doi.org/10.1103/physrev.2.329
[17] O. Kubaschewshi, E. L. L. Evans, C. B. Alcock, Metallurgical Thermo-chemistry, fourth ed. Pergamon Press[M]. Oxford, UK, 1979: 408-420.
[18] Kang Youn-bae, The Uniqueness of a Correction to Interaction Parameter Formalism in a Thermodynamically Consistent Manner[J]. Metallurgical and Materials Transactions B, 2020, 51(2): 795-804.
https://doi.org/10.1007/s11663-020-01792-1
[19] MIEDEMA A-R, DECHTEL P-F, DEBOER F-R. Cohesion in alloys—fundamentals of a semi-empirical model[J]. Physica B+C, 1980, 100(1): 1-28.
https://doi.org/10.1016/0378-4363(80)90054-6
Nickel-base superalloy;electron beam melting;evaporation behavior;activity and activity coefficient
DOI: 10.48014/pcms.20231010001
Citation: